We are testing two vaccine candidates at our Australian Centre for Disease Preparedness (formerly Australian Animal Health Laboratory or AAHL) in Geelong, Victoria.
Updated 24 April 2020
After months of research, we have a major update on our COVID-19 work. We have started pre-clinical trials for two vaccine candidates. This is happening at our Australian Centre for Disease Preparedness (ACDP) in Geelong, Victoria.
The work — in which we play a critical role — involves deep collaboration within Australia and overseas and forms part of a global rapid response to this pandemic.
Pre-clinical trials to get the vaccine faster
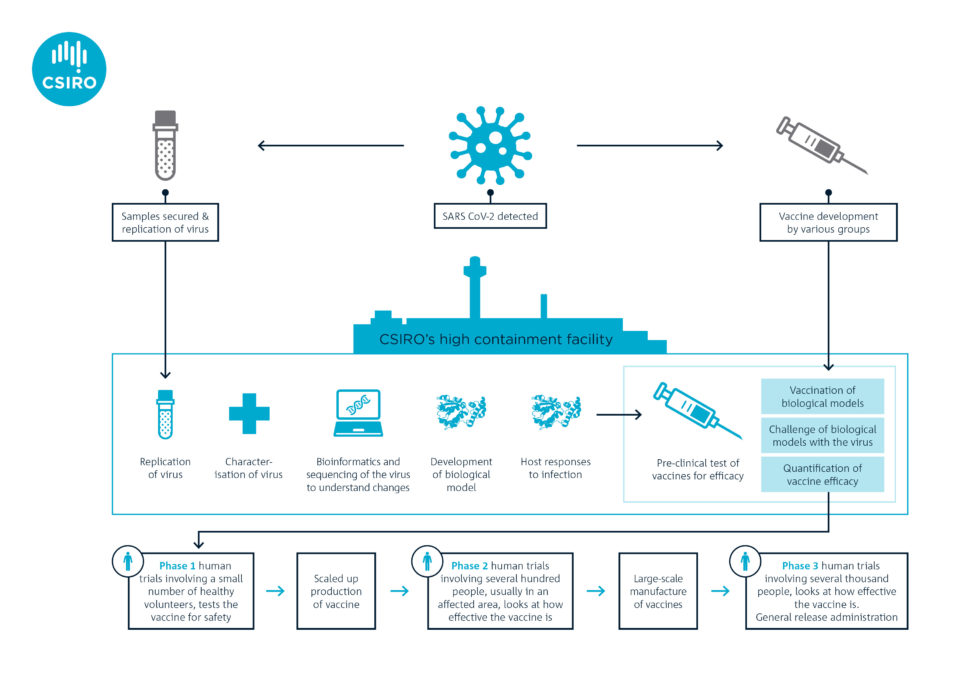
Vaccines generally take 10-15 years to develop and test. But researchers like us are hoping to streamline this process. We’re working as part of a global alliance with the Coalition for Epidemic Preparedness Innovations (CEPI). The goal is to speed up traditional vaccine development and testing and have at least one vaccine ready within 10-15 months.
CEPI asked us in January to start getting a better understanding of COVID-19 while the outbreak was in its early stages. We were the first research organisation outside of China to generate enough stocks of the virus (using the VIC01 strain isolated by the Doherty Institute) for preclinical research.
In February, we became the first team in the world to show that ferrets are susceptible to this novel coronavirus. We established the ferret model in record time and were tasked by CEPI in March to start the world’s first multi-vaccine efficacy study in animals.
In consultation with the World Health Organization (WHO), CEPI selected two candidates based on readiness for testing. The vaccine candidates come from Inovio Pharmaceuticals in the US and the University of Oxford. They’re the second and third vaccines in the world to undergo pre-clinical trials. The former is a DNA vaccine, a novel approach because there are currently no licenced DNA vaccines for humans. The latter is what’s called a vectored vaccine (an established technology).
Pre-clinical trials are underway at our high containment facility, the Australian Centre for Disease Preparedness (ACDP)
Phase 1 clinical trials
Both candidates are now entering Phase 1 clinical trials in healthy humans to see if they are safe. However, before seeking approval for Phase 2 and Phase 3 clinical trials, vaccine developers need efficacy data from pre-clinical studies. This is where organisations such as ours play a major role.
The two vaccine candidates are undergoing pre-clinical trials in our laboratory. Ferrets will be vaccinated and monitored for four weeks so it develops immunity. The ferrets will then be given a dose of the virus to measure the success of the two vaccine candidates. Such studies are planned very carefully with significant oversight of animal ethics and welfare considerations.
We’re not directly involved in developing vaccine candidates for COVID-19. But our preclinical trials are critical in helping to refine possible vaccination strategies. For example, we will test less invasive routes of vaccination (e.g. via the nose) and a two-shot regimen. The findings from our studies can have a huge impact on clinical delivery.
What else is happening at CSIRO?
We studied SARS-CoV-2’s genome sequence and confirmed the virus is changing into distinct clusters. We’re now looking at how this may impact the development and evaluation of COVID-19 vaccines, therapeutics and diagnostic tests.
In partnership with The University of Queensland, our researchers have successfully demonstrated the presence of SARS-Cov-2 in Australian untreated wastewater. This is the first step in developing an early warning surveillance system to track COVID-19 prevalence in the community.
We’re also working with the Australian Government and Victorian manufacturers to build local capability and supply of materials. This will rapidly address the demand for medical materials. And with one vaccine (developed by the University of Queensland), we are helping to scale-up production.


16th April 2020 at 11:48 am
I would like to be a vaccine trial candidate
16th April 2020 at 11:51 am
Hi Kerrie – thanks for your message.
Our work at the Australian Centre for Disease Preparedness (ACDP) is focussed on determining the characteristics of the virus, such as how long it takes to develop, how it impacts on the respiratory system and how it can be transmitted. CSIRO is not conducting human trials.
The University of Queensland is set to begin clinical trials of a potential treatment against COVID-19 (using two existing drugs, an HIV medication and an anti-malaria drug) in hospitals across Australia.
Down the track, once a COVID-19 vaccine is developed, if you’re interested in joining relevant clinical trials these links might be helpful:
The Australian New Zealand Clinical Trials Registry is a register of clinical trials being undertaken in Australia and New Zealand.
http://www.anzctr.org.au/
This US site listing clinical trials in the US and in other countries, including Australia: http://www.clinicaltrials.gov/
For general information about clinical trials in Australia: http://www.australianclinicaltrials.gov.au/
Kind regards,
Kashmi
Team CSIRO
16th April 2020 at 11:45 am
Good work team. Looking forward to seeing your results.
16th April 2020 at 11:37 am
God go with you people, I hope there is one, what a challenge .
12th April 2020 at 8:19 am
I think you are doing a sensational job, i am wondering how to apply to programs on human testing for any corona vaccines, I would really like to help.
16th April 2020 at 11:51 am
Hi Martin – thanks for your message.
Our work at the Australian Centre for Disease Preparedness (ACDP) is focussed on determining the characteristics of the virus, such as how long it takes to develop, how it impacts on the respiratory system and how it can be transmitted. CSIRO is not conducting human trials.
The University of Queensland is set to begin clinical trials of a potential treatment against COVID-19 (using two existing drugs, an HIV medication and an anti-malaria drug) in hospitals across Australia.
Down the track, once a COVID-19 vaccine is developed, if you’re interested in joining relevant clinical trials these links might be helpful:
The Australian New Zealand Clinical Trials Registry is a register of clinical trials being undertaken in Australia and New Zealand.
http://www.anzctr.org.au/
This US site listing clinical trials in the US and in other countries, including Australia: http://www.clinicaltrials.gov/
For general information about clinical trials in Australia: http://www.australianclinicaltrials.gov.au/
Kind regards,
Kashmi
Team CSIRO
4th April 2020 at 12:51 pm
Would it be possible to test animal efficacy and human safety in parallel, to further streamline the process? Or is “animal safety” a prerequisite and part of the efficacy testing stage, but not specifically shown in the graphic?
23rd April 2020 at 4:08 pm
Hi Tim, thanks for your message.
As we are in extraordinary times, some groups are choosing to conduct pre-clinical studies in parallel with Phase 1 human trials. However, Phase 2 and 3 human trials can only take place following the pre-clinical studies. At CSIRO we’re balancing the need to fast-track this work while maintaining the highest safety and quality assurances.
Kind regards,
Kashmi
Team CSIRO