We have developed a metal membrane that could accelerate the roll-out hydrogen fuel cell vehicles, and potentially develop a new export market for Australia.

Photo of CSIRO researcher in hydrogen lab.
Dr Michael Dolan in our hydrogen lab.
It’s colourless, odourless, the most abundant element in the universe, and may one day take you from 0-100 on the highway. It seems as though hydrogen is a pretty logical choice for clean fuel of the future. The kicker is that there’s very little pure hydrogen to be found anywhere on Earth, meaning we need to somehow produce it.
There are a couple of different ways to produce pure hydrogen – it can be extracted from natural gas, though carbon dioxide is a by-product. There’s also a renewable option through the electrolysis of water, which produces hydrogen and oxygen. Forcing this reaction requires a fair amount of energy which could potentially come from a clean source, like solar.
Then there’s the matter of transporting that pure hydrogen to the places it’s needed, and if we’re planning a hydrogen-powered vehicle revolution, that means every service station! Because of its low density, hydrogen can be difficult to transport and must be pressurised, and then carried by pipeline, tanker or some other secure method. While hydrogen is already being used around the world, the existing transport infrastructure is not enough to support widespread consumer use. As a standalone hydrogen delivery system, this isn’t shaping up to be cost or energy efficient.
But rest assured there are other options … ammonia for example. Ammonia is a compound of nitrogen and hydrogen that is already transported far and wide for use in industry (as fertiliser, cleaner, etc). What if we could piggyback this existing infrastructure and transport the hydrogen within the ammonia, and then extract the hydrogen from the ammonia at, or near, the point we need it?
We have spent many years researching the best ways to separate pure hydrogen from mixed gas streams, but in this case we’re separating high-purity hydrogen from ammonia. For this very purpose, we’ve developed a thin metal membrane that allows hydrogen to pass, while blocking all other gases.
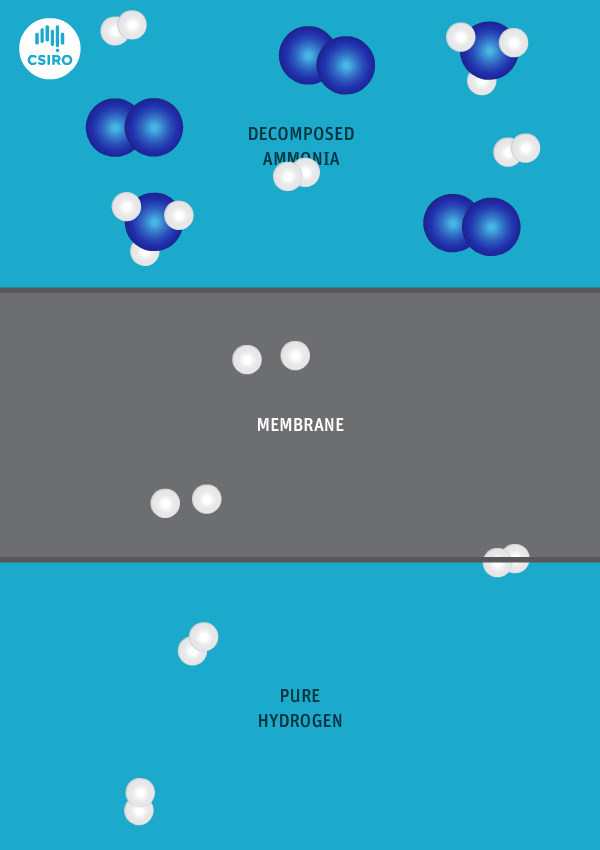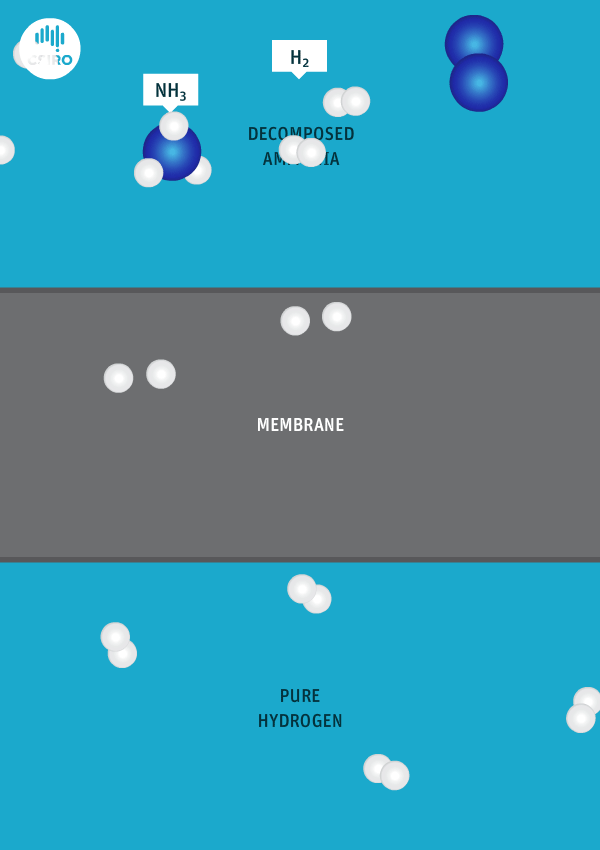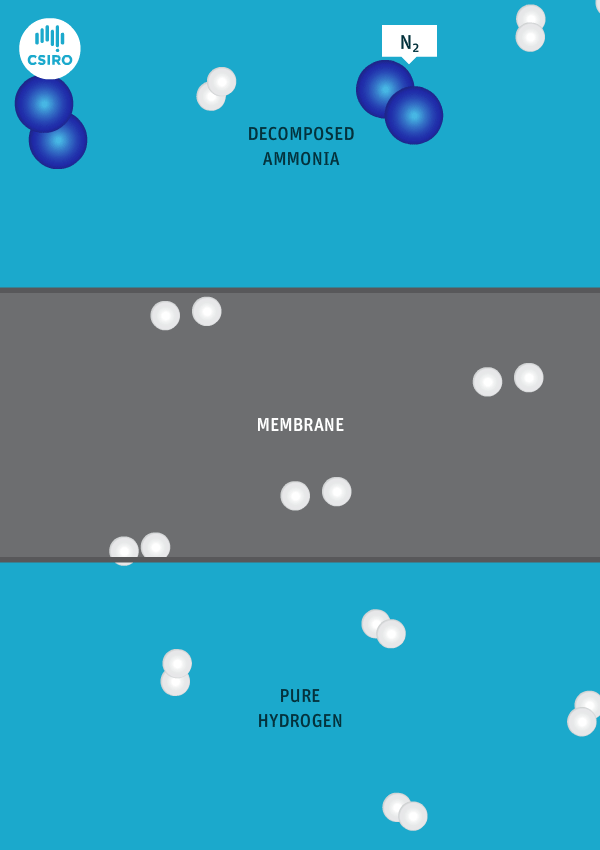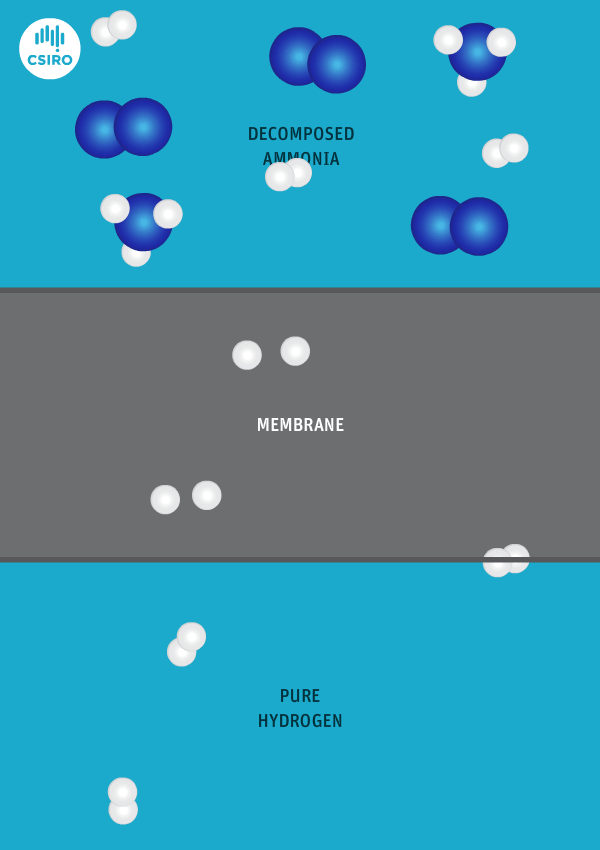
An animation shows ammonia molecules passing through a membrane to become hydrogen.
Decomposed ammonia passes through our membrane, becoming pure hydrogen.
Our membrane means that hydrogen can be transported in the form of ammonia (which is already being traded globally), and then reconverted back to hydrogen at the point of use.
While Australia is a relatively small hydrogen market, the fuel can be distributed to emerging markets in Japan, South Korea and Europe using existing infrastructure. Thinking big, we could transport Australian-made ammonia around the world so that international fuel cell vehicles could run on our hydrogen. And if we’re creating the hydrogen renewably with solar power, we are essentially exporting Australian sunshine! How’s that for home-grown ingenuity?
Our Chief Executive Dr Larry Marshall is excited by the prospect of a growing global market for clean hydrogen, and the potential for a national renewable hydrogen export industry.
“This is a watershed moment for energy, and we look forward to applying CSIRO innovation to enable this exciting renewably-sourced fuel and energy storage medium a smoother path to market,” said Dr Marshall.
Our membrane has been welcomed by industry and is supported by BOC Gas, Hyundai, Toyota and Renewable Hydrogen Pty Ltd. The project also recently received $1.7 million from the Science and Industry Endowment Fund (SIEF), which will be matched by us.
In addition to our new membrane, we’re looking forward to applying our expertise to all stages of the hydrogen technology chain (including solar photovoltaics, solar thermal, grid management, water electrolysis, ammonia synthesis, direct ammonia utilisation via combustion and/or fuel cells, as well as hydrogen production).


8th June 2017 at 10:44 am
This is a silly idea. There is more hydrogen in a litre of hydrocarbon fuel that in a litre of liquid hydrogen and the petrol or diesel is easier to store. Vehicles powered by hydrogen fuel cells often produce the hydrogen on board from a hydrocarbon for this very reason. Ammonia is made by reaction of hydrogen with nitrogen at high temperature using a iron catalysis. The water gas reaction is used to make the hydrogen by reacting steam with usually methane. It makes no sense to do this twice for no clear advantage. Has any body looked at the efficiency of this process. We should be improving the efficiency of fuel cells that use methanol or building better batteries. Neither ammonia of hydrogen are easy gases to handle.
7th June 2017 at 7:32 pm
Hydrazine is possibly the best way of fuelling transport vehicles as I pointed out in a letter to ” Oxford Today “, the O.U. magazine for alumni especially. A Japanese company has already found a non-precious metal catalyst for a fuel cell using hydrazine. Shell long ago demonstrated an electric car powered by such a fuel cell but that cell had a precious metal catalyst.
7th June 2017 at 4:55 pm
Liquid NH3 can be safely transported with high energy density at near surface temperature and pressure in tanks or by pipeline. If it leaks it can be smelt long before it is dangerous. In contrast, hydrogen has to be at high pressure and if it leaks it can cause explosions. So ammonia is both safer and cheaper to transport especially as a fuel in motor vehicles. And it can be produced readily from the hydrolysis of water from solar energy and combination of the resulting hydrogen with nitrogen from the air. It is a proven technology. There are international websites and associations dealing with this technology.
19th May 2017 at 7:11 pm
Yes ammonia can be a liquid more suitable for transportation than Hydrogen gas, but considering the atomic weight of N:14 and H:1, and the transition between 2 NH3 N2 + 3 H2 then you need to transport 34kg of ammonia to move 6kg of hydrogen just to expel the remaining 28kg of Nitrogen back to the atmosphere. The entire idea is a representation of how eager the current and highly centralized energy industry is to maintain their monopolies. Renewable energy is distributed by nature and this is a game changer that cannot be stopped, nor helped, by adding even more inefficiency to the already inefficient hydrogen value chain.
19th May 2017 at 4:41 pm
You state:
“Our membrane means that hydrogen can be transported in the form of ammonia (which is already being traded globally), and then reconverted back to hydrogen at the point of use”.
Unfortunately the NH3 “container” for H2 energy is 5.7 x the mass of the regenerated product with no use for the rejected byproduct. Not conducive to efficient transport.
7th June 2017 at 11:50 am
The main constraint with hydrogen transport today is volume, rather than weight. Compared to diesel fuel, hydrogen has 3x the energy density by weight. Transporting it via ammonia would reduce this to 50% of that for diesel. Not too bad, considering I’m using a hydrocarbon fuel for comparison. If the idea is to export renewable energy from Australia there really aren’t many options. And it would be far superior to using batteries for grid-level storage, to the point where seasonal storage may actually be viable.
7th June 2017 at 1:00 pm
The Ammonia is light compared with the heavy high pressure containers in which H2 would have to be transported at 20-30 times the pressure required to maintain NH3 in a liquid state.