As the Great Barrier Reef comes under increasing pressure from the degradation of water quality and climate change, what can we do to protect the world's largest coral reef system for future generations?

Those of us who have been fortunate enough to have travelled to spectacular coral reefs marvel at their colour and biodiversity.
At around 2,000 km long, the Great Barrier Reef is the largest coral reef system in the world. It includes 3,581 individual reefs and an immense lagoon. But the likelihood of future generations being able to enjoy the beauty of the Great Barrier Reef is dwindling, as it comes under increasing pressure from the degradation of water quality and climate change.
Warming water is one of the greatest threats facing the reef in the long term. But what about another consequence of rising carbon dioxide, ocean acidification?
When carbon dioxide dissolves in water it (slightly) increases the water’s acidity, or lowers its pH. This affects the ability of marine creatures such crustaceans, corals and coralline algae to build their skeletons. But exactly how it will affect the whole reef ecosystem is unknown.
In research published in Nature Communications, we mapped parts of the reef that are most exposed to ocean acidification. As you’d expect, there will be some regions more strongly affected than others, indicating where we might focus our efforts to preserve the reef.
Building skeletons
Conditions in the marine tropics are becoming less friendly for coral. Coral bleaching, cyclones, outbreaks of pest species and nutrient-impacted river run-off are now regular events that impact coral reef health.
What’s more, and perhaps more ominously, as the world’s oceans take up more carbon dioxide, it becomes harder for corals to secrete and maintain their calcium carbonate skeletons. While the exact response remains unknown, at some point thresholds will be reached at which dissolution exceeds calcification, leading to overall coral loss.
But ocean acidification doesn’t affect the whole reef equally. Corals change the chemistry of the seawater around them. In fact, corals are constantly building and dissolving their skeletons, taking up and releasing calcium carbonate into the water, thus increasing or lowering the pH.
The fine balance between these processes changes over the course of the day. Ocean circulation, as well as photosynthesis and respiration of other non-calcifying marine organisms, also determine the overall variability in pH of water above reefs, and therefore a coral’s ability to produce and maintain their structure.
While scientists have researched these effects on individual reefs, how do they play out on the thousands of reefs that make up the entire Great Barrier Reef?
To find the answer we used a new information system developed for the Great Barrier Reef. We found that some inshore reefs experience a lower pH now than is projected for offshore reefs in the future.
Which reefs are most threatened?
On the Great Barrier Reef, the ability for coral to build skeletons tends to decrease towards the coast. This is a consequence of the lower pH, and more nutrients, fresh water and sediment coming from the land.
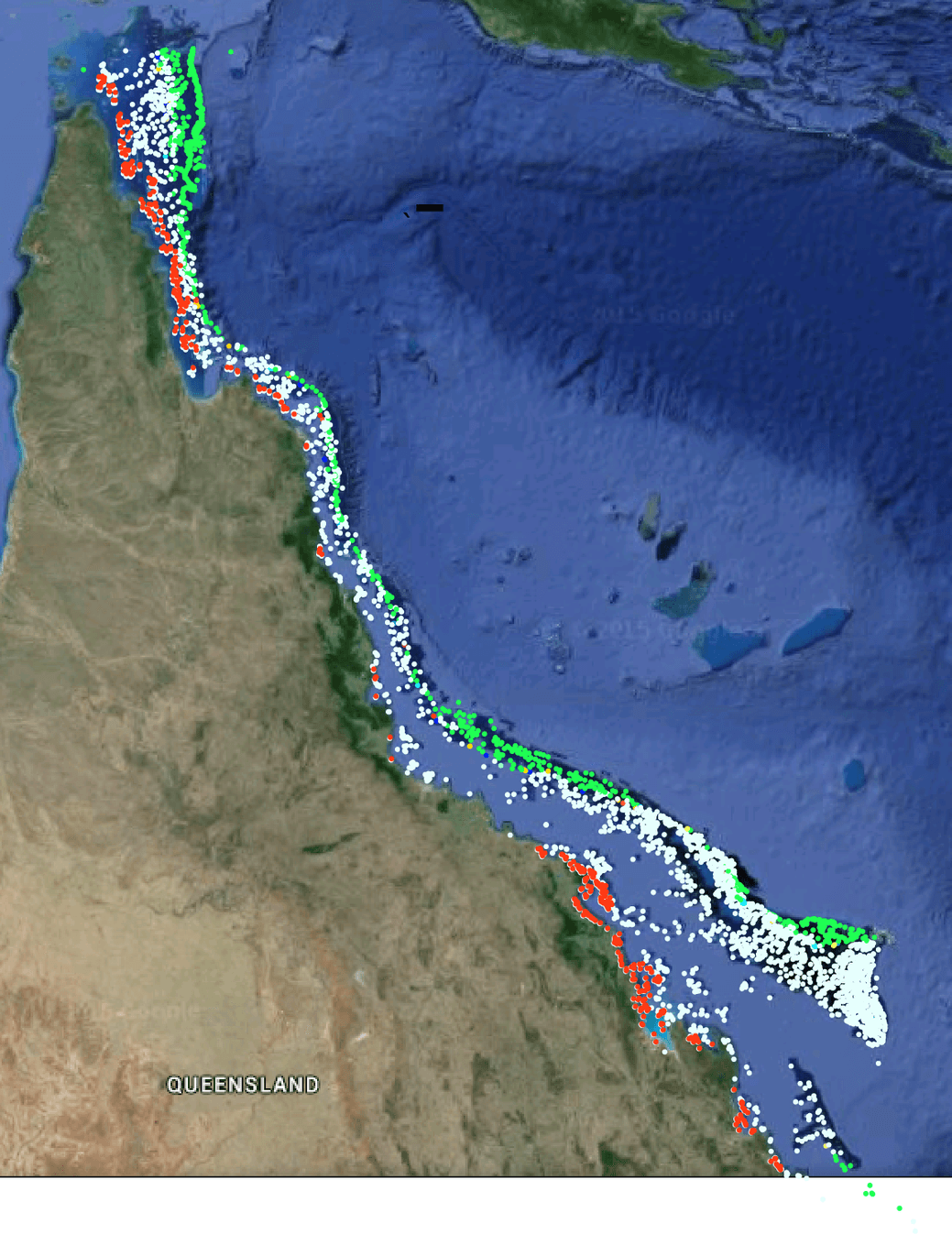
GBR Coral reef’s exposure to global ocean acidification, green reefs have some protection, white are neutral and red are already exposed.
But details of a more complex picture emerged from the study, highlighting the interaction between the thousands of reefs.
The outer reefs generally have Coral Sea water flowing over them, and for a thin band, especially in the north, their ability to build skeletons is actually driven by large scale oceanographic processes. But as the outer reef corals build their skeletons, the water flowing off them has lowered pH (more acidic). Circulation carries this water onto parts of the inner reefs, changing the average pH above their corals.
In other words, good coral health in the outer reefs, especially in the northern and southern regions, creates less favourable conditions for the mid lagoon central reefs.
What can we do?
While atmospheric carbon dioxide concentrations are increasing, focus should shift to conserve parts of the Great Barrier Reef and its corals which can be achieved through changes in the way we manage the reef. The new map of pH on the Great Barrier Reef presents the exposure to ocean acidification on each of the 3,581 reefs, providing managers with the information they need to tailor management to individual reefs.
Thus we see the Great Barrier Reef is not a singular reef nor a physical barrier that prevents exchange between reefs; it is a mixture of thousands of productive reefs and shallow areas lying on a continental shelf with complex oceanic circulation.
We cannot treat the Great Barrier Reef as one entity. We cannot summarise the impact of global ocean acidification as one number, and we cannot have one management strategy (aside from cutting global carbon emissions) to protect it.
![]()
Mathieu Mongin, Biogeochemical Modeller, CSIRO; Andrew Lenton, Senior Research Scientist, CSIRO Oceans and Atmosphere Flagship, CSIRO; Jennifer Skerratt, Coastal and enivronmental modeller, CSIRO, and Mark Baird, Team leader, Coastal and Environmental Modelling, CSIRO
This article was originally published on The Conversation. Read the original article.


1st March 2016 at 4:32 pm
Dear pjcarson2015
The carbon chemistry equilibrium in the ocean is indeed well known (but I would not call it simple ), and yes there is plenty of Ca2+ in the ocean,
to explain more clearly :
so calcium carbonate (coral and other calcifier shells) is made of Ca2+and CO32- following:
CaCO3 Ca2+ + CO32−
the carbon chemistry thermodynamic equilibrium tells us :CO32- concentration is reduced when pH drop, that is basic carbon chemistry
so as more CO2 gets absorbed in the ocean, pH drop and CO32- concentrations drops therefor CaCO3 concentrations drops as well
have a search for carbonate equilibrium in google !
while still oversaturated (from a chemical point of view i.e CaCo3 will not disappear immediately as pH drops), but the drop in CO32- is enough to reduce calcification rate , indeed coral reef and living organism are no chemist, and many experiments in the literature show that coral and other calcifiers have lower calcification rate when CaCO3 concentration is reduced, (have a look at a nature paper published last week http://rdcu.be/gAui
calcifying organisms continuously precipitate and dissolved their carbonate shell in a complex set of biogeochemical processes (again back-up by observations) so a slight modification of the equilibrium mean they can easily dwell.
Ipjcarson2015 should present himself, so no to offend the research community, before putting wrong statement in the CSIRO blog
individual should refrain from commenting about basic knowledge, distracting the discussion from it main topic, which is the variability of pH across the Great Barrier Reef and the fact that it is larger that the current projections for climate change.
2nd March 2016 at 12:25 pm
1. My credentials are as on my site – which you obviously have not read.
2. I already read your Nature reference previously.
3. [BTW: your reply is difficult to read.]
4. What “wrong statement” have I made?
5. Your statements are qualitative only – which is insufficient and leads to the wrong conclusions.
I’ve done the quantitative bit. It uses routine 1st Year uni chemistry.
6. The calculations show the spread of pH is greater than can be achieved by atmospheric CO2 spread, and so it follows that something else is causing these pH changes. This is explained in Chapters on Cyclone Pam and Ocean pH.
7. You have assumed, without testing, that CO2 causes the measured effects on shell calcification (and pH changes). That is my complaint about your article. My site shows the true natural cause for both pH and shell problems. (Look at what it is upstream from the Great Barrier Reef.)
8. Why do you refer to high-pressure-stable aragonite rather than the lower pressure calcite?
4th March 2016 at 3:18 pm
8: yes Aragonite is metastable and is thus commonly replaced by calcite in fossils, but sorry living coral are no fossil yet ! and sorry aragonite is the CaCo3 mineral used by Corals
7: “You have assumed, without testing, that CO2 causes the measured effects on shell calcification (and pH changes)” sorry but this is called ” ocean acidification”, testing of what ?,this is backup by hundred of peer reviewed literature
6: ?
5: Well what do you mean ? we used a quantitive model and observations.
4 :“wrong statement” = “Therefore, shells, corals, etc will not corrode with any feasible pH drop or atmospheric CO2 level”, again read the literature
1: “My credentials are as on my site – which you obviously have not read.” is it https://pjcarson2015.wordpress.com ?nothing there, no peer reviewed article ? there is no email, no background, education ? who you work for and so on ;..
This is my last reply to that topic,
5th March 2016 at 3:35 am
Why do we not hear about the negative effects of mankind’s massive global aquatic thermal contribution city sewers, industry along interconnected water ways, the massive cruse ships, navel vessels, and the countless other vessels that contribute volumes of thermal waste into the open waters. Weapons testing, nuclear power plants, and the many other human related sources. The oceans have a predominant inwards direction of conduction, which traps man’s contribution below the solar heated surface layer, causing a global thermal accumulation. It is this thermal accumulation that has neutralized vast areas of this very conductive value within the colder regions of the planet where it is most fragile and vulnerable to any such thermal increase, triggering the rapid bottom up decline in the Arctic and Antarctic ice. Though CO2’s are partly responsible, my own research suggests that it is mankind’s direct aquatic thermal contribution that it mainly responsible for this condition. Proven by the sheer depth of the thermal increases, which the effects of atmospheric carbon never could have reached, and the fact that experts have already openly admitted that the rate of decline was over ten times their own CO2 related predictions. A rapid rate only duplicated by experiments inclusive of the oceans natural conduction value being neutralized in areas surrounding the ice.
I can’t help but ponder the chemicals and waste humanity has been dumping into the oceans, potentially contributing to the problems with the coral reefs. My limited research doesn’t include such matters, but does suggest that it is the natural tidal flows that brings the contaminated waters of the northern hemisphere down into the southern hemisphere and back again. It is this action that I believe triggered the temporary increase in the Antarctic’s surface ice layer, due to the increased cooling effect created within the northern hemisphere, generated by the rapid decline in the Arctic’s ice structure. Now that this cooling bubble has all but been absorbed and the Arctic has a substantial reduction in its cooling potential, the globe should be witnessing a spiking up in aquatic temperatures, due to the imbalance to confront the thermal contribution of the sun, especially being tainted by man’s constant unnatural contribution. Sadly my research also strongly suggests that the Antarctic will also so substantial bottom up declines in the coming decades, which could spell disaster for the reefs around the globe, simply due to the lost cooling potentials. I’ve tried sharing my findings and concerns with others but n one cares to listen, guess everyone sees mankind’s aquatic thermal contribution and aquatic waste as being minor in comparison to the effects of the atmospheric carbon increases. I personally feel we cannot accomplish much unless we address both human related contributions aggressively. I also feel that this can be done in a cost effective manner, due to the substantial cooling effects created by massive solar structures within desert regions and on the open waters of tropical regions, where cold-spotting, shading, and venting techniques have been proven effective at generating a cooling effect, as does reforestation and increased vegetative efforts where possible. Some desert regions can even grow plants under elevated solar structures due to the shade created by the structures where water is available. But knowing the sheer size and scope of this growing condition, some have suggested that mankind invest in a major habitation effort upon the ocean surface, as being the only substantial means to blocking out the suns rays from the waters within key tropical areas, where large individual interconnected glass structures being stacked upon one another would support such an effort and support the growing of vegetation upon the surface layer to help convert the suns light energy into plant energy while also shading the surface of the waters over a vast area thus compensating for man’s aquatic thermal contribution to some small degree. If prove effective at any level, it might encourage further expansion and development. The condition seems to great for mankind to address, but I personally don’t think so. The idea of such a structure being constructed upon the ocean surface in calm tropical regions where the ocean temperatures have reach over 95 degrees might trigger a substantial cooling effect upon the waters below it, exposing quality venting potentials that could have substantial benefit if scaled up. These engineers believe that they can construct such a durable floating structure that can be added to or taken away from, as needed, to either support deep soil to grow trees, support buildings, of even lakes of surface waters designed to absorb the suns solar energy if needed. From what I’ve seen it seems possible, especially using glass, since sand is available world wide.
7th March 2016 at 11:02 am
TO THIS SITE’S CUSTODIANS.
Mongin’s point “1.” in his 04Mar16 statement defames me in that he recklessly claims I was lying. This site is publishing that defamation.
My 04Mar16 reply showed his error. Any reason why it is not shown?
7th March 2016 at 1:53 pm
Hi PJ,
As your previous comment was published on Friday we needed time to review it, as with all comments left on our blog. Because of this there can be a slight delay before anything appears on the site, especially if it’s over a weekend.
Furthermore, the current discussion is a) departing from the original topic of the post and b) becoming personal in nature.
For this reason we will not be approving any further comments.
For further information please refer to our comments policy: https://blog.csiro.au/comments-policy/
Regards,
Ellen
CSIRO Social Media
26th February 2016 at 4:34 pm
The fears expressed by the authors concerning the effect of CO2 upon ocean pH and thence on the dissolution of calcium carbonate in corals, oysters, etc are not supported by thermodynamic calculations. These calculations really should have been done before their project started; would have saved a lot of time and money!
I’ve presented the fairly routine calculations in Chapter, Ocean pH, “Planet Earth Climate Topics” on my site at pjcarson2015.wordpress.com, where you can read …..
“Comparing the Ca2+ levels (table 2) shows the much higher – 10x – typical ocean value of 0.01 (Table 1), it can be seen that the oceans are already greatly over-saturated with [Ca2+]aq. Therefore, shells, corals, etc will not corrode with any feasible pH drop or atmospheric CO2 level, so that falling carbonate levels have zero effect.”