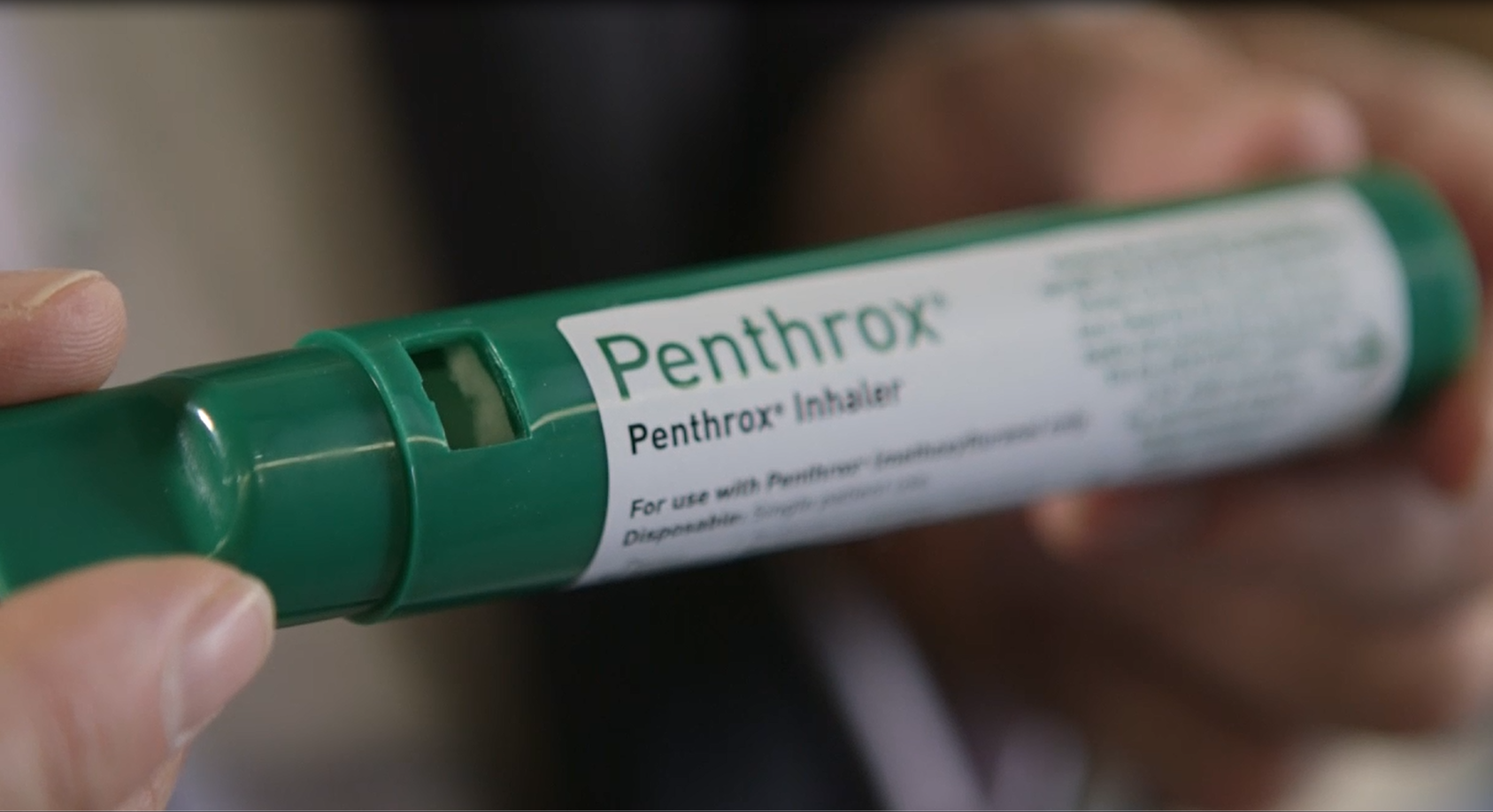
The Penthrox inhaler, nicknamed the ‘green whistle’, is an emergency pain relief device used by hospitals, surf life savers, ambulances, the defence forces and national sporting leagues.
This story is part of our spotlight series on #CSIROhealth. From apps to 3D printing, global epidemics to preventative wellbeing, we’re working in many ways, across many industries, to keep you healthy. More on our website.
We can be proud of a lengthy list of homegrown Aussie inventions that have ‘made it’ overseas, helping to improve people’s everyday lives. Around the world, the comfy ugg boot is ensuring toes stay warm and dry, WiFi is keeping everyone connected, while the bionic ear is enabling hundreds of thousands of deaf people to hear.
Now, another iconic Australian product is about to head across to foreign shores to offer a helping hand to life savers.
After getting the green light from regulators for its sale in Europe and the UK, Australian healthcare company Medical Developments International’s (MDI) is set to take the emergency pain killer Penthrox – aka the ‘green whistle’ – to the rest of the world.
Entering these huge overseas markets is a company-making achievement for MDI and we’re proud to say we’ve been integrally involved in the journey. Together we’ve developed a new manufacturing process that MDI will use to increase their production of Penthrox by ten times and meet the anticipated demand of the European market.
CSIRO’s John Tsanaktsidis has worked closely with MDI to help them upscale production of the ‘green whistle’ and enter new export markets.
Made in Melbourne, Penthrox has been used across Australia for more than 30 years, administered by hospitals, surf life savers, ambulances, the defence forces, national sporting leagues and other services in emergency situations.
What’s so unique about Penthrox? It’s administered through the unique ‘green whistle’ device which is held by the patient and inhaled via the mouth – literally sucked on – bringing immediate pain relief in a way that’s safe and non-addictive.
MDI has been granted initial regulatory approval to sell the product in the UK, France, Belgium and Ireland where Penthrox will meet a significant gap in the marketplace for a non-narcotic analgesic.
There are more than 50 million accident and emergency hospital attendances each year in these countries alone and MDI has estimated that these markets for Penthrox are worth about $100 million per year.
The new smart manufacturing process significantly reduces the cost to manufacture Penthrox’s key ingredient, the drug methoxyflurane, and provides much more consistent results.This will secure MDI’s competitive advantage on the world stage as the only global manufacturer of methoxyflurane.
Our partnership with the company goes back more than 15 years and shows how long-term research collaborations can help companies grow and become world leaders in their respective markets.
Read more about our work with MDI and some of the other health technologies we’ve developed.



18th May 2015 at 11:19 pm
A group of researchers from Czech Republic led a study in which they investigated importance companies give to innovation in relation to globalization of market in which they occur.
http://www.ibimapublishing.com/journals/JIBBP/2012/245013/245013.html
10th May 2015 at 10:06 am
Good on them for this but sad to say Ugg is now owned by a US company