Diamonds may be the ultimate in glitz for their beauty and unparalleled sparkle, but for us, the real diamond gems captivating our attention are invisible.
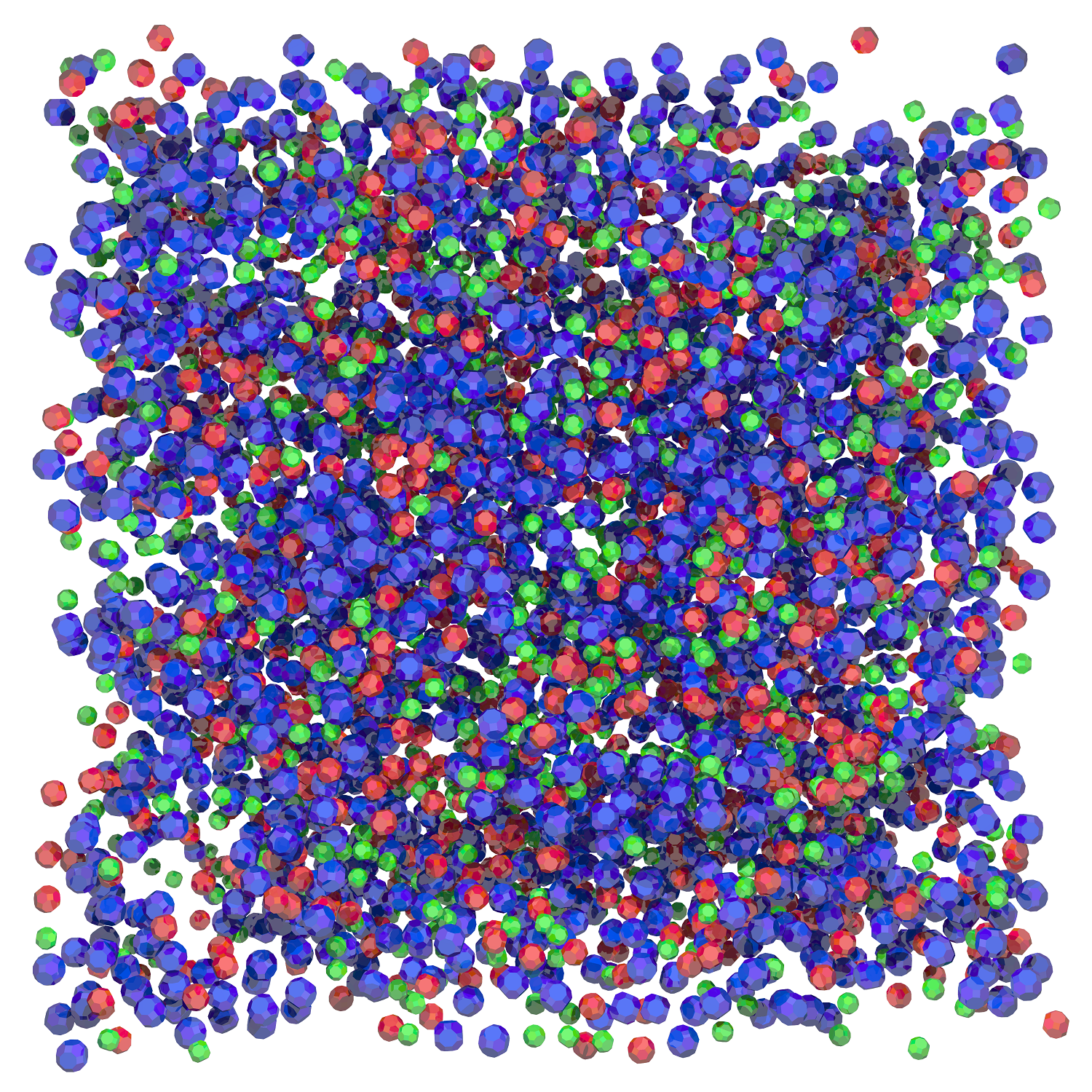
It might look like a handful of Nerds candy, but this close up of nanodiamonds shows how they form unique structures that can protect and deliver drugs more efficiently.
Diamonds may be the ultimate in glitz for their beauty and unparalleled sparkle, but for us, the real diamond gems captivating our attention are invisible.
Tiny diamond nanoparticles (or nanodiamonds) – only an 8000th the width of a human hair – are proving to be extremely valuable in medicine, giving way to new life saving treatments and diagnostic tests.
Just a couple of years ago, a diamond discovery by our virtual nanoscientist and current Feynman Prize winner, Amanda Barnard, underpinned a new chemotherapy treatment that targets brain tumours.
Developed by the UCLA (University of California, Los Angeles), the treatment uses nanodiamonds to carry chemotherapy drugs directly into brain tumours, providing greater cancer-killing efficiency and less side effects than other treatments.
The technology was made possible thanks to Amanda’s discovery that diamond nanoparticles have unique electrostatic properties that repel or attract (kind of like a magnet) so that they spontaneously arrange into very useful structures.
Let’s take a closer look at what that looks like. Nanodiamonds have many facets – imagine the polished cut of a precious gem or the surface of a soccer ball – and each facet has an electrostatic property characterised by a different colour.
The coloured surface of one nanodiamond connects with the complementary colour surface of another nanodiamond, while surfaces of the same colour repel. Multiply this process by many and the particles come together much like a three-dimensional jigsaw puzzle.
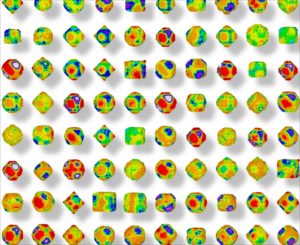
The coloured facets of each nanodiamond have a unique electrostatic property that allows them to attract or repel.
It’s these electrostatic properties that the UCLA used to bond the chemotherapy drug, doxorubicin, to the nanodiamonds at a molecular level.
The nanodiamond-doxorubicin combination (or NDX) delivers the same drug but with greater precision, in reduced doses, and with a slower and sustained release. It has been shown to delay tumour growth and improve patient outcomes.
Nanotechnologies like this offer huge potential in medicine, and when employed side by side with pharmacology, will improve drug delivery methods and speed up drug discovery.
For example, we can move away from repeated, re-administration of treatments and use more implants that offer the same (or better) control over dose rate and treatment cycles.
New drugs that more effectively target the site of disease will also lead to reduced dosages and less side effects, as well as the ability to tailor treatments.
We hope that our nanoscience research will continue to influence breakthrough health developments that benefit people around the globe.


27th June 2015 at 9:45 pm
I knew there was a deeper reason why diamonds are a girl’s bestfriend.