Trilobites graced the Earth for 270 million years until they were wiped out in the ‘Great Dying’. Image Credit: http://news.bbc.co.uk/2/hi/in_pictures/8398477.stm
Around 250 million years ago, an extinction event took place that was unprecedented in its size and scale. Known colloquially as the ‘Great Dying’, the Permian Triassic extinction event wiped 90 percent of species (both marine and terrestrial) forever from the map. It is the largest recorded mass extinction event in Earth’s history, and was estimated to have set biological evolution back by tens of millions of years.
There are many theories as to the cause of this Great Dying, ranging from giant meteor impacts to massive volcanic eruptions. In a paper published today in Nature Geoscience, a team of our researchers have supported the case for a much tinier – yet no less fascinating – contributor to the kill: methane-producing bacteria, fed from the bowels of the earth.
Silent but deadly
During this ancient era, massive methane-producing bacterial blooms, nourished by volcanic atmospheric nickel, are thought to have disrupted the carbon cycle and released toxic levels of methane and carbon dioxide – resulting in a runaway greenhouse effect on the Earth’s atmosphere.
But how did these levels of nickel come to be released into the atmosphere? Rock records have revealed massive volcanic eruptions occurred during this period, yet the notion that nickel would be released into the atmosphere during eruptions was not widely believed by scientists who study magmas and volcanoes.
Our research team, led by Dr Stephen Barnes in collaboration with Prof. James Mungall from the University of Toronto, are proposing that metals like nickel, which are normally concentrated at the bottom of magma chambers, hitched a ride to the atmosphere on the back of vapour bubbles, also forming rich ore deposits simultaneously with the ancient bacterial blooms.
Raised on heavy metal
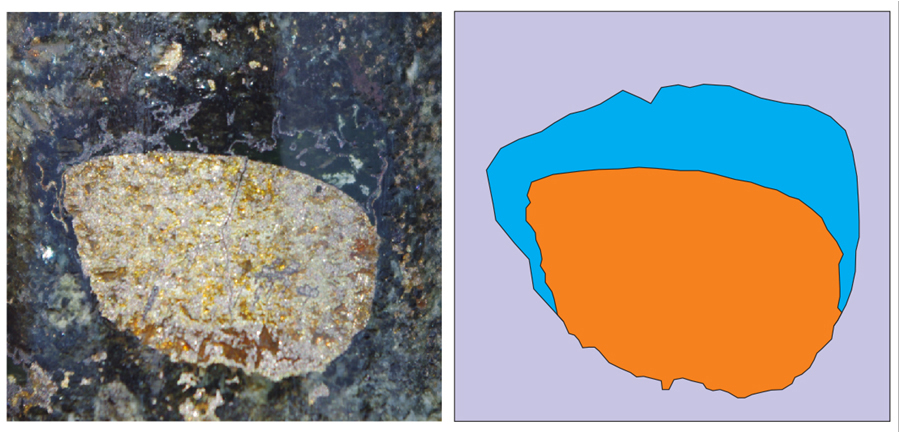
A solidified sulphide liquid droplet (orange in diagram) with a silicate “cap” now recognised to be an infilled gas bubble (blue in diagram) from the Kharaelakh nickel deposit, Siberia, which was active during the Great Dying.
Magma deep within the Earth’s crust commonly carry droplets of sulphur-rich melts that contain metals. But these sulphide melts are dense and would be expected to sink to the bottom of the magma reservoir.
But the ‘vapour transport mechanism’ proposed by our researchers can explain how these dense metal sulphide melts are able to be found at shallower depths than expected.
‘In the lab we found that small droplets of the sulphide melt can attach to the vapour bubbles and use the buoyancy of the bubbles to float upwards,’ Steve said.
‘Even more interesting for us was the discovery that this transport mechanism provides a theoretical link between our understandings of how the magmatic and hydrothermal processes of metal ore formation from magma overlap .’
The paper, Transport of metals and sulphur in magmas by flotation of sulphide melt on vapour bubbles, is available online from Nature Geoscience.
For media enquiries, please contact Keirissa Lawson | Keirissa.Lawson@csiro.au | M: 0418 282 055



4th March 2015 at 9:20 pm
And we are just waiting for the next CH4 burp…
25th February 2015 at 11:30 am
Keirissa. Thanks, but your reference is to the abstract – much as reproduced above. The authors substantiate their theory with laboratory experiments. I doubt they reproduced geological pressures so that substantiation is dubious.
My difficulty is: how do vapour bubbles form under the immense pressures at depth required by this mechanism?
Is it possible that such nickel deposits form more simply by diffusion (over a very long time)? When the nickel ore reaches the higher cooler levels, it gets trapped by crystallisation, ie it’s no longer mobile. This process would be similar to zone refining as used to purify semiconductors.
13th March 2015 at 10:07 am
There are actually many experiments that have produced volatile-saturated (bubble-bearing) mafic magmas with reasonable water and CO2 contents at high pressure (just search Google scholar for combinations of water saturated basalt melting high pressure) but that’s not really important here. We know that the Norilsk ores formed in the presence of a free vapour phase, because we can see evidence for it in the presence of infilled gas bubbles in the rocks and ores. That’s what the picture in the blog post shows. The Norilsk ores are unusual in that they formed at very shallow depths in the crust (probably less than 1 km). Most nickel deposits formed much deeper than that. and those don’t show any evidence for a vapour phase. So the presence of vapour bubbles is not a necessary condition to make most nickel sulphide ores, which form by the universally accepted mechanism of accumulation of sulphide liquid out of deep-seated basaltic magmas. The mechanism we are proposing in the paper only operates at relatively shallow levels. Hope that answers your question.
24th February 2015 at 3:14 pm
How do VAPOUR droplets form at the immense pressures near the bottom of the magma?
24th February 2015 at 3:57 pm
It’s basically like opening a champagne bottle. The amount of gas that the magma can dissolve is greater at higher pressure, so when you take the lid off (i.e. bring the magma closer to the surface) the gas can’t stay dissolved any more and comes out as bubbles. Like taking the cork out of the champagne bottle…
24th February 2015 at 4:35 pm
I realise that. But your article stated the vapour droplets dragged the nickel from the BASE of the magma, ie nowhere near the surface, and there are no bubbles at that pressure.
[I expect the gases are not actually “dissolved” but chemically bound as sulphides, carbonates, etc – unstable at those temperatures but the high pressures at depth drive the equilibrium to prevent dissociation.]
Anyway, I doubt there are any such bubbles in magma until it ventures very near the surface – perhaps a few kilometres – and then giving the “champagne effect”. For example, “smokers” are only found AT the Ridges where magma actually is at or near the surface. You theory would say that bubbles would also be detected near the Ridges. Also volcanic seeps (such as PNG discussed by Fabricius) are very restricted in area – again, only where magma is very close to the surface.
[If they existed, one should be able to detect such bubbles and where they were, with instrumentation.]
25th February 2015 at 10:20 am
Hey Peter, if you would like to go into more technical detail, we urge you to check out the original paper: Transport of metals and sulphur in magmas by flotation of sulphide melt on vapour bubbles by J. E. Mungall, J. M. Brenan, B. Godel, S. J. Barnes & F. Gaillard.