Our scientists have developed a way to make more environmentally friendly fuels.
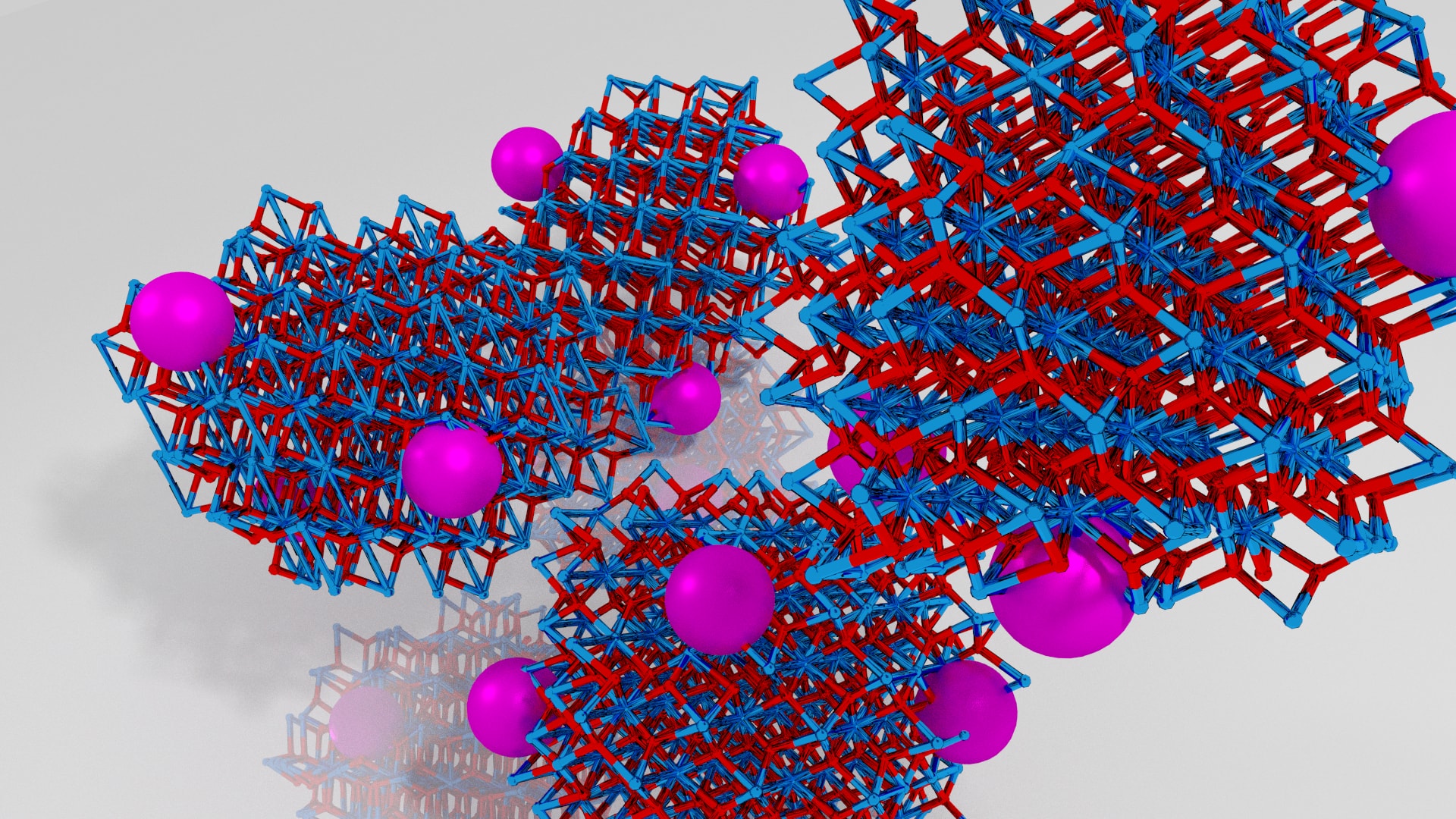
Illustration of reactor (transparent): CO2 and H2 (top) pass through the catalyst bed (black plug) where it reacts and transforms into methane and water (bottom). Credit: Renata Lippi
Artist’s rendition of our fantastic catalyst (not 100% accurate). Purple are Ruthenium nanoparticles supported on the ZrO2 nanoparticles (Zr – blue, O – Red). In reality they are in the nanometer range 10-20 nm and are black. Credit: Renata Lippi, University of Adelaide
When we hear the word ‘environment’ it conjures images of the natural world – vast forests, rolling hills, pristine waterways and fresh air… but these wonders are under threat from greenhouse gases produced by industrialised activity and fossil fuels. For years scientists have been searching for ways to sustain and protect these natural resources so they can continue to support future generations long after we’ve all checked out.
Like all good stories, there is a baddie in the sustainable environment tale, hell-bent on thwarting our science crusaders. Anthropogenic carbon dioxide (CO2) emissions, the product of combusting fossil fuels, plays this role. When fossil fuels that are solid (coal) or stored underground (petrol and natural gas) are burned to provide energy, they produce CO2 in a gaseous form which is released directly into the atmosphere. And we release a lot of it! We also use natural gas, a fossil fuel that contains mostly methane (CH4), for a lot of our industrial activities.
So how do we solve this problem? How can we have our industrialised world and breathe clean air too?
One obvious way is to make more environmentally friendly fuels, and our scientists, working closely with researchers at University of Adelaide have developed a way to do this!
They have created a new catalyst that efficiently converts CO2 into a synthetic form of natural gas. This is particularly exciting because CO2 is a very inert (unreactive) molecule, and has historically been difficult to transform into a commercially useful chemical. But this challenge didn’t stop our researchers. Their new way of synthesising a very efficient catalyst produces almost pure methane from CO2.
‘So what’s a catalyst?’ We hear you ask.
Catalysts are very important materials in the chemical industry. They decrease the activation energy of chemical reactions, allowing reactions to occur which otherwise wouldn’t. In this case, the CO2 methanation used CO2 and H2 (hydrogen) as reactants to produce CH4 and water.
The synthesis process developed by the researchers uses an incredibly interesting next gen material called metal organic frameworks, or MOFs. Made of metals joined to each other by organic linkers, the tiny MOF crystals are full of molecular-sized holes that can absorb, store, separate and release just about anything. Now hold on, because this gets technical… In this instance, the holes of a zirconium-based MOF were filled with a solution of a ruthenium (Ru) salt (just like a sponge, when you add a few drops of water, the sponge quickly absorbs it by spreading it into its holes) so the Ru salt would be well dispersed within the MOF. The dried MOF with Ru acted as a sacrificial template to form Ru nanoparticles well dispersed over ZrO2 nanoparticles. Still with us? Great! These nanoparticles make a remarkably active catalyst.

Illustration of reactor (transparent): CO2 and H2 (top) pass through the catalyst bed (black plug) where it reacts and transforms into methane and water (bottom). Credit: Renata Lippi
Not only does this highly active catalyst have the potential to help us solve the problem of CO2 emissions on earth but it could also help in the Mars program. Mars’ atmosphere is 96% CO2, and with SpaceX’s Elon Musk recently announcing it will launch a crewed mission to Mars, they plan to produce methane (fuel) on Mars via the same reaction.
The researchers had to synthesise and screen over one hundred materials before they discovered Ru-loaded MOFs provided the optimum results. To speed up this process the team used the rapid automated materials and processing (RAMP) capability. The state-of-the-art high-throughput equipment located at our Clayton site helped fast track materials discovery and process development by running automated experiments, much faster than through traditional methods. It was also discovered the catalyst can operate at low pressures and mild temperatures, which makes it perfect for potentially running on solar energy.
Although H2 itself can be used as fuel, it is difficult to store and transport due to its flammability although we are working on that problem too. But by producing H2 from renewable (or ‘green’) processes like solar, wind energy and combining with CO2, the resulting synthetic natural gas is safer to handle and can use existing natural gas infrastructure. This concept is called “power-to-gas”.
With an abundance of CO2 available in the atmosphere, this exciting new catalyst for recycling it has enormous potential to replace fossil fuels. What’s more, because it may use ‘clean’ solar energy to do so it doesn’t increase atmospheric CO2 in the process.
We all know that high CO2 levels in our atmosphere is bad for our planet and contributes to global warming, and that it will take a number of different technologies to get us to a lower emissions future. Although it sounds like an oxymoron, synthetic natural gas could be one key answer to a cleaner, greener industrial world, thanks to MOFs and RAMP.
To read more about this exciting work, head to the Journal of Materials Chemistry A.


4th July 2017 at 5:07 pm
This assumes a supply of CO2, and it is in our interests to avoid any CO2 in the first place. It looks like a variant on ‘clean coal’, technically feasible but not viable economically. A hydrogen economy, although technically more challenging, is an environmentally benign target. Maybe that’s where your research should be. Also ethanol is a fuel that can be derived via organics from atmospheric CO2 so is environmentally neutral and well understood – there are vehicles and aeroplanes already using it. That also needs research to make it more effective (less land use etc.)
3rd July 2017 at 12:55 pm
And water is also produced as a by-product! Is this a possible new industry using South Australia’s abundant wind generated power?
2nd July 2017 at 3:12 am
Unbelievably fascinating
How can you invest in this story
30th June 2017 at 3:19 pm
but CH4 is also a greenhouse gas, and if it is used as a fuel it produces more CO2 as a product!
30th June 2017 at 1:20 pm
This seems like a more sustainable take on the water shift reaction used to make town gas, Could I suggest that rather than converting the CO2 to burnable fuel (OK for specialist uses) the main thrust of the project should be to go a step further and use the product syngas as a raw material for making polymers and possibly other materials that could lock up CO2 for a long period and also be useful? This could help lower the concentration of CO2 in the atmosphere. It might even be retrofittable to existing power plants exhausts?
I doubt it will make coal burning any cleaner, since CO2 is not the only pollutant from coal burning, but at least it is better than pumping captured CO2 back underground, which begs the question “why dig up the coal in the 1st place?”!