Our new algorithm maps new blood vessel growth with more fidelity to quickly zero in on early-stage cancers.
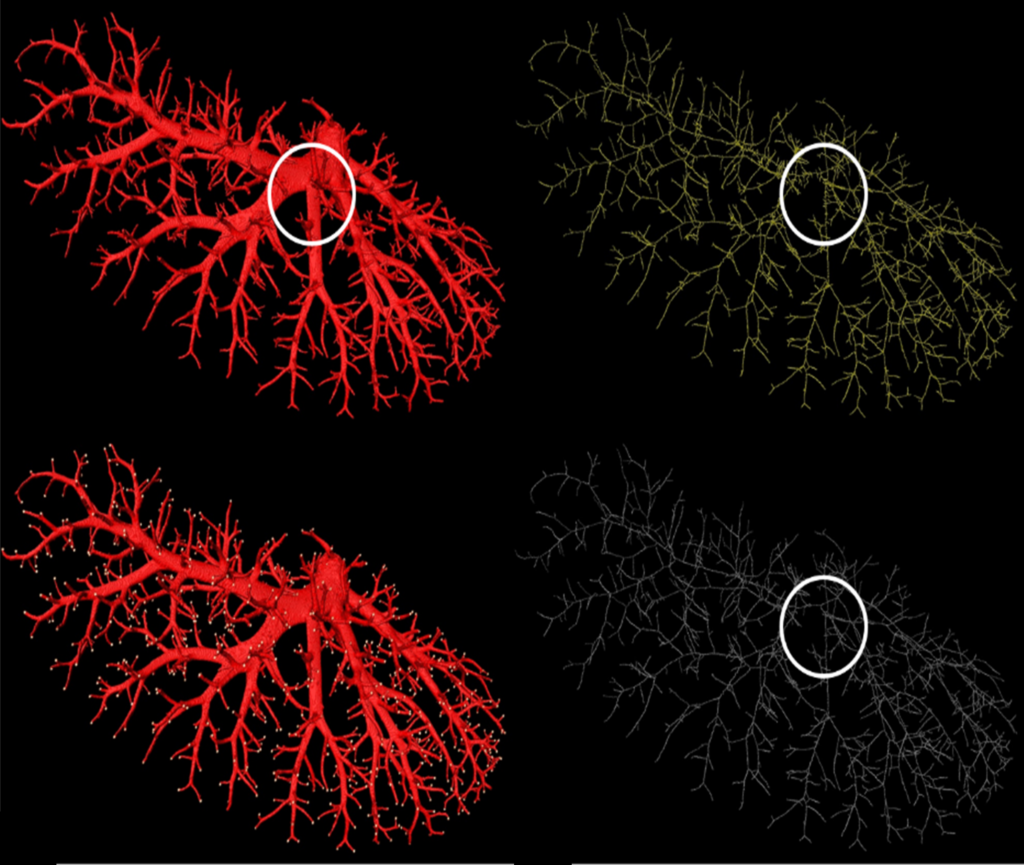
(Top left) The hepatic vein of a rat; (top right) The skeleton generated by the ITK 3D thinning method; (bottom left) End points of the terminal vascular branches; (bottom right) The skeleton generated by our method.
To grow, cancerous cells feed on a constant supply of nutrients from blood vessels. Like piping infrastructure preceding a new development, the growth of new blood vessels can be mapped to locate early-stage cancers quickly — and with our new algorithm, those maps are finer and more accurate than ever before.
The cells that cling together in tubes and globes, bones and noses to form the cellular mass that is you — they’re each a little bit like a fire. Give them a feed of fuel and oxygen, and they release heat and grow. Remove that feed, either fuel or oxygen, and they die away. This is true for normal and abnormal cells alike.
Tumours are abnormal cells unable to control their own growth, are out of control, ‘rogue’, and can manifest into malignant tumours, cancer, which can grow and split, circulating around the body to create ‘spot fire’ metastases.
The good news is, tumours, like regular cells, need a constant supply of blood, which carries the nutrients and oxygen needed for the tumour to thrive and divide. If tumours receive no blood supply, they remain a harmless cluster of cells incapable of growing larger than 1 or 2 mm3, or, the size of a sprinkle.
So how do tumours go from innocuous flesh sprinkles to life-threatening growths?
Angiogenesis.
Angiogenesis is the creation of new blood vessels that deliver nutrients and oxygen to the growing tumour, and remove its waste products — the piping infrastructure. Angiogenesis occurs all the time, but tumour growths can kick-start this process around them to fuel their fire. Like screeching baby birds, tumours release various ‘signal proteins’, which tell the body to produce new vessels and blood to shroud the growth in a nutrient feed, helping the cells to grow.
By understanding angiogenesis, and modelling blood vessel growth accurately, researchers can detect nascent tumour growth and stamp it out. In a ‘where there’s smoke, there’s fire’ scenario, where there are newly growing blood vessels, angiogenesis, there may be tumours.
Currently, high-resolution images are taken of the area’s blood vessel structure, but — due to technical limitations — these images have some of their most vital details stripped away: the fine detail of the blood vessel tips, how many of these terminal vessels there are, and emphasising vessel volume at the cost of actual geometry.
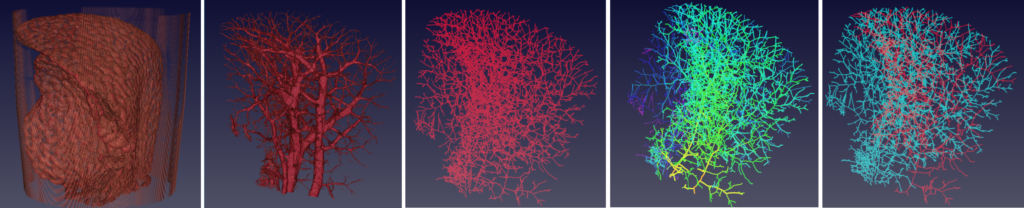
To ratchet up the quality of this imaging, our researchers paired up with the Shanghai Institute of Applied Physics, Chinese Academy of Sciences to gather micrometre-scale images of various cancer stages in the brains and livers of mice. Studying these images, the team were able to develop an algorithm that overcame the resolution drawbacks of previous techniques.
Left to right: 1) MicroCT scan, 2) binary image, 3) vessel skeletons, 4) branching statistics, 5) tree visualisation.
The algorithm, when applied to the blood vessel images, works to thin down the structure and — unlike previous methods — retain information on blood vessel length, branching patterns, and terminal points.
Like the workings of Precogs from The Minority Report, the revealed intricate tips of new blood vessels portend tumourous growths that can be apprehended before they manifest, increasing the survival outcomes of patients.
“Our robust algorithms for the early detection and quantification of angiogenesis could potentially be a great step forward in the detection and treatment of cancer,” said lead researcher Dr Dadong Wang.
Though the algorithm helps to better define blood vessel patterns, there are still obstacles that lie between these imaging techniques and their application with human patients.


16th August 2017 at 11:31 am
You are correct Peter, I don’t see it either!
Nicholas, where is the connection?
16th August 2017 at 12:10 pm
Hi Aaron,
The link to the correct blog is here: https://blog.csiro.au/scanning-for-iron-new-alzheimers-research-could-help-predict-dementia/
Cheers,
Ellen
CSIRO Social Media
4th August 2017 at 10:21 am
I was expecting the link to take me to an article on Alzheimer’s disease and iron.
4th August 2017 at 11:50 am
Hi Stephen, thanks for pointing this out. There was a hyperlink error in the email version of the newsletter. It should have directed you here
https://blog.csiro.au/scanning-for-iron-new-alzheimers-research-could-help-predict-dementia/
8th August 2017 at 10:41 am
Maybe it’s me, but I still can’t find the Alzheimer’s/iron connection.
16th August 2017 at 12:09 pm
Hey Peter,
There was a link mix up in the email, the link to this blog was accidentally put in place instead of the link to the this blog: https://blog.csiro.au/scanning-for-iron-new-alzheimers-research-could-help-predict-dementia/. Sorry for the confusion.
Cheers,
Ellen
CSIRO Social Media