We have developed a metal membrane that could accelerate the roll-out hydrogen fuel cell vehicles, and potentially develop a new export market for Australia.

Photo of CSIRO researcher in hydrogen lab.
Dr Michael Dolan in our hydrogen lab.
It’s colourless, odourless, the most abundant element in the universe, and may one day take you from 0-100 on the highway. It seems as though hydrogen is a pretty logical choice for clean fuel of the future. The kicker is that there’s very little pure hydrogen to be found anywhere on Earth, meaning we need to somehow produce it.
There are a couple of different ways to produce pure hydrogen – it can be extracted from natural gas, though carbon dioxide is a by-product. There’s also a renewable option through the electrolysis of water, which produces hydrogen and oxygen. Forcing this reaction requires a fair amount of energy which could potentially come from a clean source, like solar.
Then there’s the matter of transporting that pure hydrogen to the places it’s needed, and if we’re planning a hydrogen-powered vehicle revolution, that means every service station! Because of its low density, hydrogen can be difficult to transport and must be pressurised, and then carried by pipeline, tanker or some other secure method. While hydrogen is already being used around the world, the existing transport infrastructure is not enough to support widespread consumer use. As a standalone hydrogen delivery system, this isn’t shaping up to be cost or energy efficient.
But rest assured there are other options … ammonia for example. Ammonia is a compound of nitrogen and hydrogen that is already transported far and wide for use in industry (as fertiliser, cleaner, etc). What if we could piggyback this existing infrastructure and transport the hydrogen within the ammonia, and then extract the hydrogen from the ammonia at, or near, the point we need it?
We have spent many years researching the best ways to separate pure hydrogen from mixed gas streams, but in this case we’re separating high-purity hydrogen from ammonia. For this very purpose, we’ve developed a thin metal membrane that allows hydrogen to pass, while blocking all other gases.
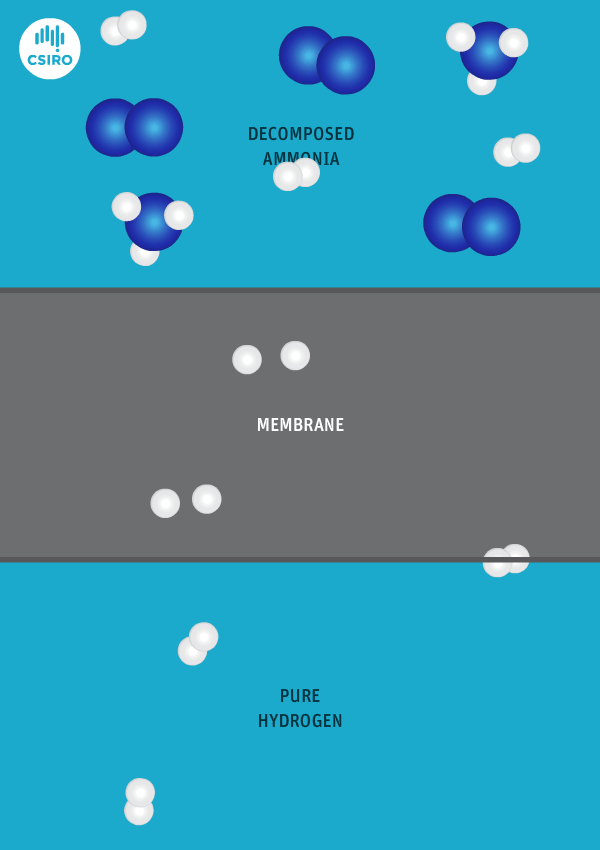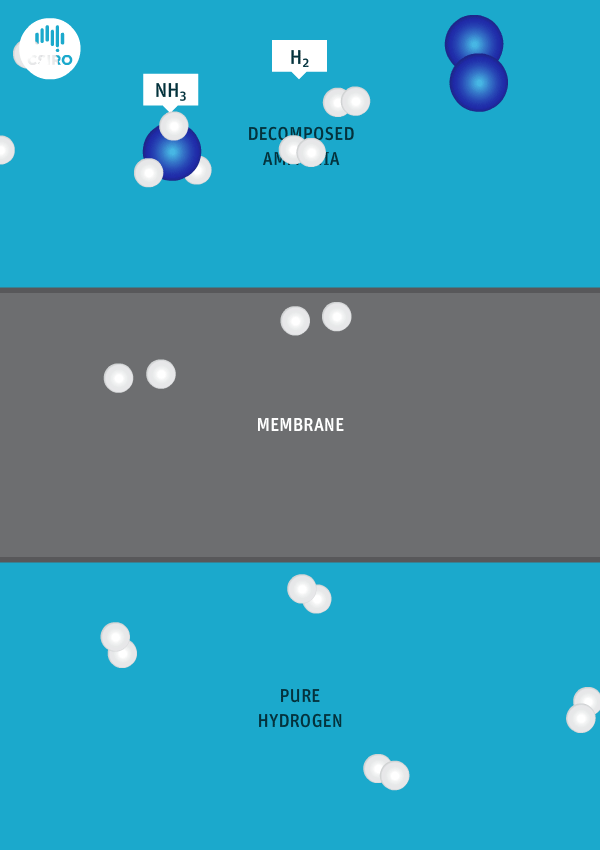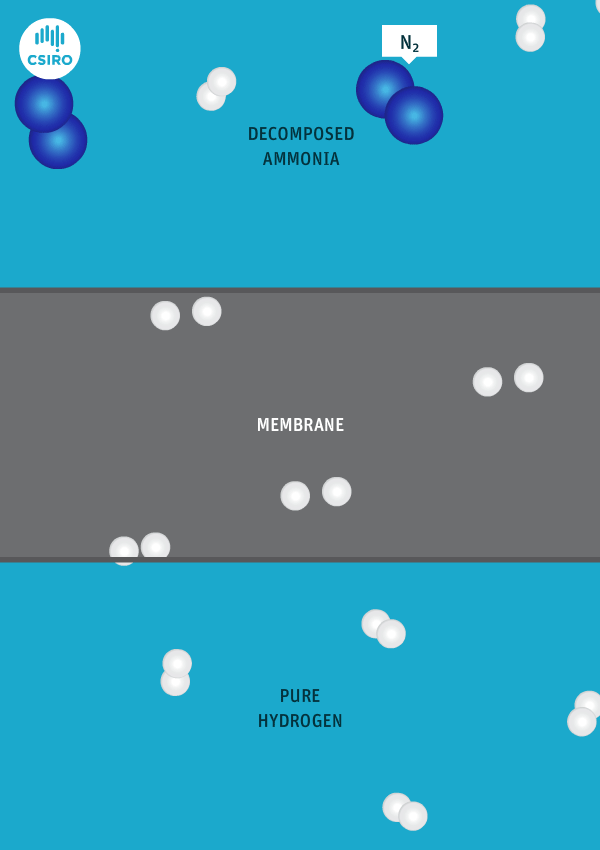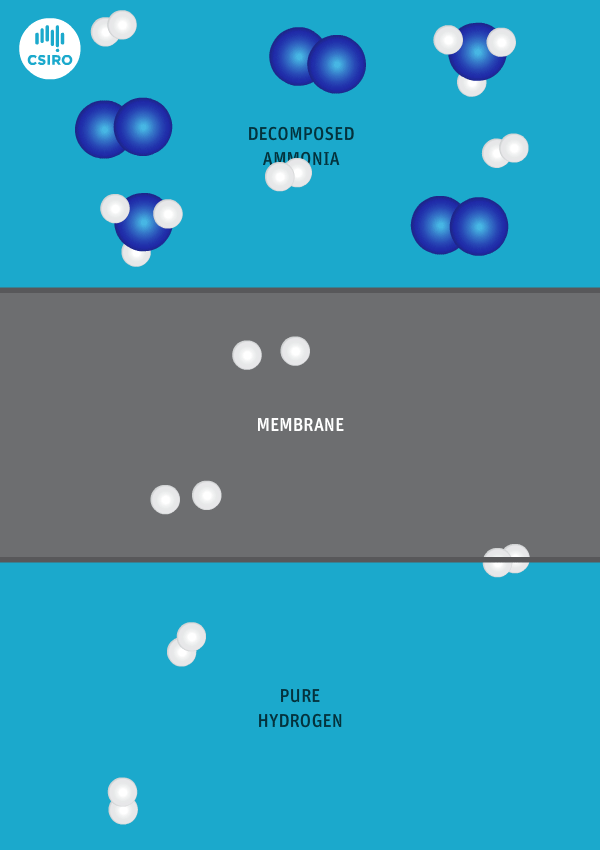
An animation shows ammonia molecules passing through a membrane to become hydrogen.
Decomposed ammonia passes through our membrane, becoming pure hydrogen.
Our membrane means that hydrogen can be transported in the form of ammonia (which is already being traded globally), and then reconverted back to hydrogen at the point of use.
While Australia is a relatively small hydrogen market, the fuel can be distributed to emerging markets in Japan, South Korea and Europe using existing infrastructure. Thinking big, we could transport Australian-made ammonia around the world so that international fuel cell vehicles could run on our hydrogen. And if we’re creating the hydrogen renewably with solar power, we are essentially exporting Australian sunshine! How’s that for home-grown ingenuity?
Our Chief Executive Dr Larry Marshall is excited by the prospect of a growing global market for clean hydrogen, and the potential for a national renewable hydrogen export industry.
“This is a watershed moment for energy, and we look forward to applying CSIRO innovation to enable this exciting renewably-sourced fuel and energy storage medium a smoother path to market,” said Dr Marshall.
Our membrane has been welcomed by industry and is supported by BOC Gas, Hyundai, Toyota and Renewable Hydrogen Pty Ltd. The project also recently received $1.7 million from the Science and Industry Endowment Fund (SIEF), which will be matched by us.
In addition to our new membrane, we’re looking forward to applying our expertise to all stages of the hydrogen technology chain (including solar photovoltaics, solar thermal, grid management, water electrolysis, ammonia synthesis, direct ammonia utilisation via combustion and/or fuel cells, as well as hydrogen production).


12th May 2017 at 2:33 pm
Always good to see useful research results coming from the CSIRO. I believe ammonia is a good candidate for a carbon free fuel as it has similar transport properties to LPG/propane, which is easy enough to handle. I do have a couple of questions regarding this particular breakthrough:
Can you explain what exactly the special feature of your membrane is? I would have thought that there are lots of materials that will let hydrogen pass and no other gasses.
Your article (and the media articles based on it) seems to make no distinction between ammonia and decomposed ammonia (i.e. mixture of N2 and H2 gasses). Isn’t this decompsition the trickier part of turning ammonia into a viable fuel source? How is that decomposition acheived? Does your membrane play any part in this process?
11th May 2017 at 10:33 pm
It is my understanding that you can mix Hydrogen with natural gas and then burn the mixture. This takes advantage of existing pipelines. It may mean that the fuel burners may need to be modified. This could also be a useful transition path toward 100% renewable. Hydrogen has more than 2X the calorific value/kg of natural gas.
12th May 2017 at 2:13 pm
I remember seeing a presentation by Siemens about five years ago where they announced that they were going to develop gas turbines for large power generators that could run on any methane/hydrogen mixture, including pure hydrogen. They were supposed to be commercially available by 2017. Haven’t been able to find any updates on that topic though.
17th May 2017 at 3:57 pm
There is a lot of effort currently into direct ammonia utilisation technologies, like turbines, internal combustion engines and fuel cells. These will complement the work that CSIRO and others are doing to extract H2 from ammonia. At a single energy hub you can generate electricity for the grid, recharge electric vehicles and produce H2 for fuel cell vehicles.
7th May 2017 at 7:59 am
Seems great concept, research and result . The trouble is the strident far right in politics that are bent on cooking the planet.
6th May 2017 at 7:04 pm
Fascinating. Would it be possible to use the same, or very similar, membrane with acidified liquids? I’m relying on chemistry I did decades ago but recall that “acids” have a lot of disassociated hydrogen in the form of H+ ions. Of course, they’d have to be paired with the missing electrons but there is bound to be ways to do that.
The future is certainly exciting. Let’s just hope we get to experience it before our reliance on fossil fuels & the like see us off!
9th May 2017 at 11:04 am
Hi there,
We passed your question on to scientist and this is his response:
“H+ present in an acidic solution won’t dissolve into metals because a charged atom can’t dissolve into a metal which has no overall charge. As you mentioned, however, if you create an electrical circuit to supply an electron to balance the positive change on H+ it can dissolve into the membrane. This is called cathodic charging, a process which occurs in metal hydride batteries, and it sometimes happens as a side-effect of electroplating. We’ve never tried to harness this as a way of extracting H2 from acidic solutions– maybe we should look at that next!”
Cheers,
Ellen
CSIRO Social Media
9th May 2017 at 6:40 pm
Isn’t the extraction of hydrogen from an acidified solution simply electrolysis?
5th May 2017 at 5:33 pm
Making ammonia is energy intensive as is decomposing it. Seems like a good transport medium if you can solve these problems without also producing CO2 in synthesizing NH4 or NOx in decomposing it.
12th May 2017 at 11:37 am
Agree.
However mother nature has worked out how to use photosynthesis as the energy source to crack that triple bond. Check out all those beautiful wattle trees. They’re doing it right now. Even your channa massala, dahl, poppadoms, hommus, tofu, soy milk or baked beans are bought to you thanks in part to photosynthesis and N fixation. It think they may also produce H2 in the process. It would be interesting to see a comparison with Haber-Bosch. Then there is also dissimilatory reduction of nitrate to ammonia which may enable us to use waste streams. A great opportunity for bio-mimicry.
13th May 2017 at 6:31 pm
See http://pubs.acs.org/doi/pdf/10.1021/ja5042836 regarding decomposing ammonia. It seems that ammonia can be decomposed using a simple
flow reactor with only a small amount of energy. The temperatures they are talking about (530°C-550°C) aren’t very high by industrial standards and could be achieved by using electricity from renewable sources.